The diagnosis and risk stratification of acute pulmonary embolism (PE) are critical steps in patient management. These processes not only influence the urgency of intervention but also the choice of therapeutic modalities. This comprehensive section aims to delve into the granular aspects of clinical prediction rules, laboratory tests, imaging modalities, and special population considerations, serving as an exhaustive resource for healthcare professionals.
Clinical Prediction Rules
Wells Criteria for PE
- Overview: The Wells Criteria is a cornerstone for assessing the pretest probability of PE. This predictive model consists of seven criteria ranging from clinical manifestations of DVT to underlying malignancy.
- Components and Scoring: The original Wells Criteria for PE includes seven elements, each with a different point value:
- Clinical signs and symptoms of deep vein thrombosis (DVT) (+3.0)
- An alternative diagnosis is less likely than PE (+3.0)
- Heart rate > 100 bpm (+1.5)
- Immobilization for more than 3 days or surgery in the previous 4 weeks (+1.5)
- Previous, objectively diagnosed PE or DVT (+1.5)
- Hemoptysis (+1.0)
- Malignancy (on treatment, treated in the last 6 months, or palliative) (+1.0)
- Interpretation: Scores are classified as follows:
- Score > 6.0: High probability
- Score 2.0 to 6.0: Moderate probability
- Score < 2.0: Low probability
- Clinical Implications:
- Low Risk (Score < 2.0): Consider D-dimer testing; if negative, PE is unlikely.
- Moderate to High Risk (Score ≥ 2.0): Further imaging studies, like a computed tomography pulmonary angiography (CTPA), are warranted to confirm or exclude PE.
Geneva Score
- Overview: The Geneva Score offers a more nuanced risk assessment, including factors such as age, previous DVT or PE, and recent surgery or fracture.
- Revised Versions: Subsequent adaptations of the Geneva Score have incorporated lab findings and arterial blood gas measurements, providing a multi-dimensional risk profile.
- Clinical Applications: Similar to the Wells Criteria, the Geneva Score guides whether to proceed with D-dimer testing or direct imaging studies.
- Components and Scoring: The Revised Geneva Score incorporates more variables than the Wells Criteria, and all are objective:
- Age > 65 years (+1)
- Previous DVT or PE (+3)
- Surgery or fracture within 1 month (+2)
- Active malignancy (+2)
- Unilateral lower limb pain (+3)
- Hemoptysis (+2)
- Heart rate 75–94 bpm (+3) or ≥95 bpm (+5)
- Pain on lower limb deep venous palpation and unilateral edema (+4)
- Interpretation: Scores are classified as follows:
- Score > 8: High risk
- Score 4 to 8: Intermediate risk
- Score < 4: Low risk
- Clinical Implications:
- Low Risk: Consider D-dimer testing; if negative, PE is unlikely.
- Intermediate to High Risk: Further imaging studies are recommended.
Pulmonary Embolism Severity Index (PESI)
The Pulmonary Embolism Severity Index (PESI) is a clinical tool used to assess the prognosis of patients with a confirmed diagnosis of pulmonary embolism (PE). It helps stratify patients into different risk categories based on the likelihood of 30-day mortality. This stratification aids in making decisions about the level of care, including considerations for outpatient treatment versus hospitalization.
Overview:
- PESI is designed to predict the risk of mortality and other adverse outcomes in patients diagnosed with PE. It takes into account various factors, including the patient’s age, comorbidities, and clinical presentation at the time of diagnosis.
Scoring and Categories:
- Scores are allocated based on the presence of certain factors, each of which is assigned a point value. The total score places patients into one of five risk classes.
- Risk Classes:
- Class I: Very low risk (Score 0 to 65)
- Class II: Low risk (Score 66 to 85)
- Class III: Intermediate risk (Score 86 to 105)
- Class IV: High risk (Score 106 to 125)
- Class V: Very high risk (Score >125)
- Each risk class corresponds to an increasing risk of 30-day mortality, from less than 1% in Class I to more than 10% in Class V.
Components:
PESI incorporates 11 objective clinical variables, each assigned a different weight:
- Age (in years; 1 point per year)
- Cancer (active: 30 points)
- Chronic heart failure (10 points)
- Chronic pulmonary disease (10 points)
- Increased pulse rate (>110/min: 20 points)
- Low systolic blood pressure (<100 mm Hg: 30 points)
- Low body temperature (<36°C/96.8°F: 20 points)
- Altered mental status (60 points)
- Arterial hypoxemia (PaO2 <60 mmHg: 20 points)
- Male gender (10 points)
- Tachypnea (respiratory rate >30/min: 20 points)
Clinical Implications:
- Patients in Class I or II are typically considered at low risk for adverse outcomes and may be candidates for outpatient treatment, provided no other contraindications are present.
- Patients in Class III to V generally require hospitalization and possible intensive care monitoring, depending on the severity of their condition and the presence of other risk factors.
The PESI score is a valuable tool in the clinical decision-making process for patients with PE, helping to guide treatment decisions and resource allocation. However, it should be used as a complement to, not a substitute for, clinical judgment.
Simplified Pulmonary Embolism Severity Index (sPESI)
- Overview: sPESI is a streamlined version of PESI, designed for easier clinical application. It focuses on six major variables.
- Scoring:
- Each variable is assigned one point, making it a binary scoring system.
- A score of 0 indicates low risk, whereas a score ≥1 suggests a higher risk of adverse outcomes.
- Components:
- Age >80 years, history of cancer, history of chronic heart failure or pulmonary disease, heart rate ≥110 bpm, systolic blood pressure <100 mmHg, and arterial oxyhemoglobin saturation <90%.
- Clinical Implications:
- Low Risk (Score of 0): These patients are generally good candidates for outpatient management with anticoagulation.
- High Risk (Score ≥1): Hospitalization and potentially more aggressive interventions should be considered.
- Utility in Pharmacotherapy:
- Like PESI, sPESI serves as a useful tool in selecting the appropriate anticoagulation regimen and setting, but it offers the advantage of simplicity for quick bedside decisions.
Comparative Utility
- When to Use Which:
- PESI offers a more detailed risk stratification and is generally used in settings where a comprehensive evaluation is feasible.
- sPESI is preferred for quick, bedside evaluations, especially in emergency settings where rapid decision-making is crucial.
- Both in Tandem:
- Some clinicians opt to use both PESI and sPESI, starting with sPESI for initial risk assessment and refining with PESI as more clinical information becomes available.
D-dimer Testing and Age-Adjusted D-dimer
- Role: D-dimer, a fibrin degradation product, serves as a sensitive but non-specific marker for PE.
- Age-Adjusted D-dimer: Recognizing that D-dimer levels increase with age, the use of age-adjusted D-dimer cutoffs (age x 10 ng/mL for patients older than 50 years) can reduce false-positive rates and avoid unnecessary imaging in older patients.
Imaging Modalities and Ultrasound
- CT Pulmonary Angiography (CTPA)
- First-Line Imaging: CTPA offers a detailed visualization of the pulmonary arterial system and is the first-line imaging modality for suspected PE.
- Utility: Beyond confirming PE, CTPA provides insights into right ventricular strain, offering indirect clues about severity and prognosis.
- Ultrasound for DVT Assessment
- Role: Given that DVT is a frequent source of PE, an ultrasound of the deep veins of the legs can provide valuable diagnostic information.
- Situations: Particularly useful in patients who cannot undergo CTPA due to contraindications like renal impairment or contrast allergy.
Pulmonary ECG
ECG findings in PE may include signs of right heart strain such as S1Q3T3 pattern, right bundle branch block, or T wave inversions in the right precordial leads. While these findings are non-specific, they may raise suspicion for PE in the appropriate clinical context.
Cardiac Biomarkers
Cardiac biomarkers such as troponin and B-type natriuretic peptide (BNP) may be elevated in PE, reflecting right ventricular strain. Elevated levels can be associated with a worse prognosis and may guide therapeutic decisions, particularly in patients with submassive or massive PE.
Special Populations and Other Prognostic Models
- Pregnant Women: Standard risk stratification models may not be entirely applicable in pregnancy. Modifications in imaging studies, like preferring lung scintigraphy over CTPA, may be considered to minimize radiation exposure.
- Patients with Cancer: PE is a common complication in patients with active malignancy. Wells and Geneva Scores may need to be interpreted cautiously, considering the pro-thrombotic state induced by cancer.
- Chronic Heart or Kidney Disease: These conditions may alter D-dimer levels and the interpretation of imaging studies, necessitating tailored risk stratification approaches.
- Other Prognostic Models:
- sPESI (Simplified PESI): A streamlined version of PESI, focusing on six major variables including age, cancer, heart failure, systolic blood pressure, pulse rate, and arterial oxyhemoglobin saturation level.
- Hestia Criteria: Incorporates clinical judgment into a list of objective criteria, offering another angle for risk stratification.
Table 1: Wells Criteria for PE
| Criteria | Points |
| Clinical signs/symptoms of DVT | 3.0 |
| Alternative diagnosis less likely | 3.0 |
| Heart rate > 100 | 1.5 |
| Recent immobilization/surgery | 1.5 |
| Previous DVT/PE | 1.5 |
| Hemoptysis | 1.0 |
| Malignancy | 1.0 |
Score > 6.0: High probability
Score 2.0 to 6.0: Moderate probability
Score < 2.0: Low probability
Clinical Implications:
Low Risk (Score < 2.0): Consider D-dimer testing; if negative, PE is unlikely.
Moderate to High Risk (Score ≥ 2.0): Further imaging studies, like a computed tomography pulmonary angiography (CTPA), are warranted to confirm or exclude PE.
Table 2: Geneva Score for PE
| Criteria | Points |
| Age | 2 |
| Previous DVT/PE | 3 |
| Surgery/fracture within 1 month | 2 |
| Active malignancy | 2 |
| Unilateral lower limb pain | 3 |
| Hemoptysis | 2 |
| Heart rate | 1 |
| Pain on lower limb deep venous palpation | 1 |
Score > 8: High risk
Score 4 to 8: Intermediate risk
Score < 4: Low risk
Clinical Implications:
Low Risk: Consider D-dimer testing; if negative, PE is unlikely.
Intermediate to High Risk: Further imaging studies are recommended.
Description: These tables present two commonly used clinical prediction rules for estimating the pretest probability of Pulmonary Embolism (PE): the Wells criteria and the Geneva score. Each criterion within these scoring systems is assigned a specific number of points. By summing the points corresponding to the patient’s clinical features, healthcare professionals can categorize the patient into different risk levels (e.g., low, intermediate, high) for PE. These risk levels guide further diagnostic testing, including the use of D-dimer tests and imaging studies like CT pulmonary angiography (CTPA). Understanding and applying these criteria can lead to more accurate and timely diagnosis and management of PE.
Graphic 1: PE Diagnostic Pathway
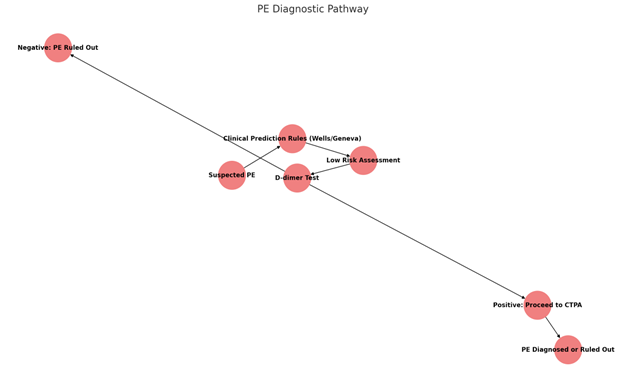
Description: This flowchart outlines the diagnostic pathway for suspected PE, beginning with the initial clinical suspicion and utilizing tools like the Wells criteria or Geneva score for risk assessment. Based on the risk level, the D-dimer test may be used to rule out PE in low-risk patients. A positive D-dimer result or higher risk assessment leads to further investigation, typically with CTPA. This flowchart serves as a visual guide for healthcare professionals to navigate the complex diagnostic process for PE, ensuring evidence-based decision-making and patient-centered care.
Table 3: Interpretation of D-dimer Test Results
| D-dimer Result | Interpretation | Action |
| Negative | PE ruled out in low-risk patients | No further testing for PE |
| Positive | Further investigation required (e.g., CTPA) | Proceed with imaging studies |
This table summarizes the interpretation of D-dimer test results in the context of PE diagnosis, including actions taken based on the results.
Risk Stratification
- Pulmonary Embolism Severity Index (PESI): Classifies patients into risk categories to guide treatment decisions, including the potential for outpatient management.
- Right Ventricular Dysfunction: Assessed by echocardiography or CTPA, it may guide therapy, including consideration of thrombolytic treatment.
Summary
The diagnosis and risk stratification of PE involve a multifaceted approach that integrates clinical prediction rules, laboratory testing, and various imaging modalities. The selection of diagnostic tests must be individualized, considering factors such as clinical suspicion, underlying health conditions, and the potential risks and benefits of each method. The accurate and timely diagnosis of PE, coupled with appropriate risk stratification, is paramount in guiding optimal therapeutic decisions and improving patient outcomes.
