Factors to Consider
1.HCV treatment history (naïve or experienced)
2.Cirrhosis (none, compensated, decompensated)
3.HCV genotype
4.Medication reconciliation (including herbal/dietary supplements) & potential drug interactions
5.Access to food
6.Adherence (one vs. three tablets; duration – 8 vs. 12 weeks)
7.Past medical history (e.g., transplant, human immunodeficiency virus)
Glecaprevir/Pibrentasvir Data
- ENDURANCE-1
- Phase 3 randomized trial of HCV genotype-1 patients without cirrhosis on glecaprevir-pibrentasvir for 8 (n=351) or 12 (n=352) weeks
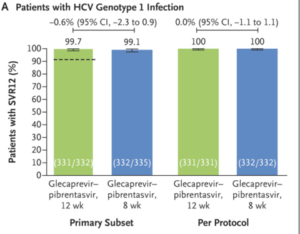
- 8 week treatment duration was non-inferior to 12 weeks
- One patient experienced on-treatment virologic failure
- Similar safety profile across both treatment duration with headaches and fatigue being most common
- No patients discontinued treatment due to side effects
- No documented relapse in either study arm
Ledipasvir/Sofosbuvir Data
- ION-1
- Phase 3 randomized trial of HCV genotype-1 patients + cirrhosis on ledipasvir/sofosbuvir OR ledipasvir/sofosbuvir + ribavirin x 12 weeks, ledipasvir-sofosbuvir OR ledipasvir-sofosbuvir + ribavirin x 24 weeks
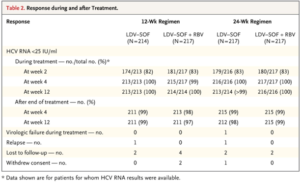
-
- No difference in SVR12 rate between those with cirrhosis (97%) vs. without cirrhosis (98%)
- Most common side effects were fatigue, headache, insomnia, nausea
- ION-3
- Phase 3 randomized trial of HCV genotype-1 patients without cirrhosis on ledipasvir/sofosbuvir OR ledipasvir/sofosbuvir + ribavirin x 8 weeks, ledipasvir-sofosbuvir x 12 weeks
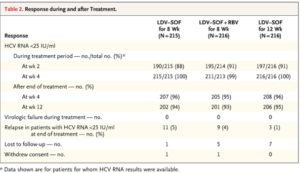
-
- SVR12 rates of 93-95% across all study arms
- Higher relapse rates with 8 week tx noticed in those who had baseline HCV RNA level <6 million IU/mL
- Most common side effects were fatigue, headache, and nausea
Download PDF:
