Treatment Goals
- Eradicate virus
- Prevent progression of liver disease and death
- Prevent hepatocellular carcinoma
- Achieve sustained virologic response (SVR) at week 12 post-treatment
- Surrogate marker – aim to have absence of detectable virus in blood
| Class | Mechanism of Action | Medications |
| NS3/4A protease inhibitor | Prevents cleavage of HCV-encoded polyprotein (into mature forms of NS3, NS4A, NS5A, NS5B proteins), essential for viral replication | Glecaprevir
Grazoprevir Voxilaprevir Paritaprevir Simeprevir |
| First generation NS5A inhibitor | Potent antiviral activity against HCV NS5A (essential for viral replication and virion assembly) | Daclatasvir
Ledipasvir Ombitasvir |
| Second generation NS5A inhibitor | Pibrentasvir
Elbasvir Velpatasvir |
|
| NS5B polymerase inhibitor | Metabolized to active uridine analog triphosphate and acts as chain terminator for NS5B polymerase | Sofosbuvir |
Hepatitis B Reactivation – Black Box Warning
- Test for current or prior HBV infection
- If HBV not treated, may result in reactivation and lead to fulminant hepatitis, hepatic failure, and death
- Measure hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (anti-HBc) before initiating HCV treatment
- Risk in HBsAg positive and in those HBsAg negative + anti-HBc positive
- In those with serologic evidence, monitor for clinical and lab signs of hepatitis flare or HBV reactivation (increase in aminotransferase levels, bilirubin levels)
Ledipasvir/sofosbuvir [Harvoni]
- Patients > 3 years old
- Once-daily tablet (adults)
- Tablets or pellets depending on weight-based dosing (children)
- Pellets must be swallowed whole
- Can be sprinkled into non-acidic soft food at or below room temperature and consumed within 30 minutes of mixing
- No dose adjustments for renal impairment including end stage renal disease (ESRD)
- No safety data in pediatric population
- Indications:
- Genotype 1, 4, 5, 6 w/out cirrhosis or w/ compensated cirrhosis
- Genotype 1 with decompensated cirrhosis + ribavirin
- Genotype 1 or 4 s/p liver transplant without cirrhosis or w/ compensated cirrhosis + ribavirin
- ADR: headache, fatigue, asthenia
Interactions
- Amiodarone = fatal cardiac arrest; bradycardia (may occur up to 2 weeks after initiating HCV treatment)
- P-gp inducers may decrease plasma concentrations
- Antacids = decrease ledipasvir concentration
- Separate administration by 4 hours
- H2-receptor antagonist = administer with or 12 hours apart
- Famotidine 40 mg PO twice daily is max dose
- Proton pump inhibitors = administer with under fasting conditions
- Omeprazole 20 mg PO daily is max dose
- Anticonvulsants, antimycobacterials, HIV antiretrovirals, HMG-CoA reductase inhibitors (statins)
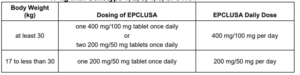
Sofosbuvir/Velpatasvir [Epclusa]
- Patients >6 years old or weighing at least 17 kg
- Tablets
- No dose adjustments for renal impairment including end stage renal disease (ESRD)
- No safety data in decompensated cirrhosis or pediatric patients
- Pangenotypic (covers all genotype)
- ADR: headache and fatigue
Interactions
- Amiodarone = fatal cardiac arrest; bradycardia (may occur up to 2 weeks after initiating HCV treatment)
- P-gp and/or moderate to potent inducers of CYP2B6, CYP2C8, or CYP3A4 may decrease plasma concentrations of sofosbuvir and/or velpatasvir
- Antacids = decrease velpatasvir concentration
- Separate administration by 4 hours
- H2-receptor antagonist = administer with or 12 hours apart
- Famotidine 40 mg PO twice daily is max dose
- Proton pump inhibitors = AVOID. If medically necessary, take sofosbuvir/velpatasvir with meal and 4 hours before omeprazole 20 mg PO daily
- No other proton pump inhibitor has been studied
Glecaprevir/pibrentasvir [Mavyret]
- Patients > 3 years old
- Three tablets daily (adults)
- Tablets or pellets depending on weight-based dosing (children)
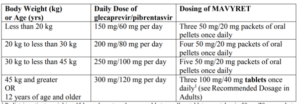
- Must be taken with food
- Pellets should be sprinkled on small amount of soft food with low water content that can be swallowed whole (e.g., peanut butter, cream cheese, Greek yogurt)
- Contraindicated in:
- Moderate to severe hepatic impairment (Child Pugh B or C) or those with history of prior hepatic decompensation
- Coadministration with atazanavir or rifampin
- Pangenotypic (covers all genotype)
- ADR: headache, fatigue
Interactions
- P-gp/CYP3A4 inducer can decrease glecaprevir/pibrentasvir plasma concentrations
- Coadministration with ethinyl estradiol-containing products may increase risk of ALT elevations
- Coadministration with HMG-CoA reductase inhibitors can increase statin concentrations
- Dose reduction
- Consider use of lower dose
- Avoid atorvastatin, lovastatin, and simvastatin
Sofosbuvir + velpatasvir + voxilaprevir [Vosevi]
- Adult patients
- 1 tablet by mouth daily with food x 12 weeks
- Not indicated in moderate to severe hepatic renal impairment (Child Pugh B or C)
- Indications:
- Without cirrhosis or compensated cirrhosis
- Genotype 1, 2, 3, 4, 5, 6 with prior treatment containing NS5A inhibitor
- Genotype 1a or 3 with prior treatment containing sofosbuvir without NS5A inhibitor
- ADR: Headache, fatigue, diarrhea, nausea
Interactions
- Amiodarone = fatal cardiac arrest; bradycardia (may occur up to 2 weeks after initiating HCV treatment)
- P-gp and/or moderate to potent inducers of CYP2B6, CYP2C8, or CYP3A4 may decrease plasma concentrations of sofosbuvir + velpatasvir + voxilaprevir
- Antacids = decrease velpatasvir concentration
- Separate administration by 4 hours
- H2-receptor antagonist = administer with or 12 hours apart
- Famotidine 40 mg PO twice daily is max dose
- Proton pump inhibitors = administer with using omeprazole 20 mg PO daily max
- No other proton pump inhibitor has been studied
- Anticoagulants, anticonvulsants, antimycobacterials, antiretrovirals, HMG-CoA reductase inhibitors
Ribavirin
- Used in combination with decompensated cirrhosis and in certain clinical scenarios (e.g., genotype 3 with prior sofosbuvir-based treatment failure)
- Weight-based, split-dosing and administered with food
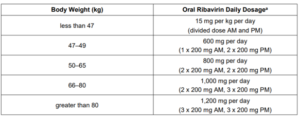
- ADR: anemia, cough, insomnia, dyspnea, pruritus, rash, nausea