Phenobarbital for Acute Alcohol Withdrawal: Prospective Randomized Double-blind Placebo-controlled Study.
Rosenson et al. J Emerg Med. 2013 Mar;44(3):592-598
| Objective | To investigate if a single dose of intravenous (i.v.) phenobarbital combined with a standardized lorazepam-based alcohol withdrawal protocol decreases intensive care unit (ICU) admission in ED patients with acute alcohol withdrawal |
| Design | Prospective, randomized, double blind, placebo-controlled study. |
| Population | 198 patients with suspected acute alcohol withdrawal syndrome |
Design
| Inclusion/Exclusion | I: > 18 year old with suspected acute alcohol withdrawal syndrome E: Allergy to study drugs, hepatic impairment, no IV access, and other primary diagnosis |
| Intervention | IV phenobarbital (10 mg/kg) in 100 mL normal saline over 30 mins |
| Outcomes | Primary: Initial level of hospital admission from the ED Secondary: Use of continuous lorazepam infusion, hospital length of stay, total amount of lorazepam used, and incidence of adverse events |
Baseline Characteristics
| Phenobarbital (n = 51) | Placebo (n = 51) | |
| Male | 46 (90) | 45 (88) |
| Age, years: median (IQR) | 46 (40–52) | 48 (37–54) |
| Initial AWCA score: median (IQR) | 6 (4–10) | 7 (4–10) |
| Initial heart rate: median (IQR) | 106 (100–123) | 112 (108–120) |
| Initial tremor: n (%) | 48 (95) | 48 (95) |
| Initial sweats: n (%) | 25 (49) | 32 (63) |
| Initial agitation: n (%) | 20 (40) | 21 (41) |
| Initial anxiety: n (%) | 35 (68) | 43 (84) |
| Altered level of consciousness: n (%) | 30 (58) | 35 (68) |
| Auditory/visual disturbances: n (%) | 20 (40) | 21 (41) |
| Time to initial lorazepam administration, minutes: median (IQR) | 84 (48–146) | 84 (40–312) |
| Time to study medication administration, minutes: median (IQR) | 144 (103–263) | 150 (100–26 |
| Patients with prior alcohol withdrawal admissions to study institution: n (%) | 21 (41) | 25 (49) |
Results
| Primary Outcome | ICU admission rate: Phenobarbital vs Placebo8% vs. 25% Difference 17% [95% confidence interval (CI) 4–32%]) |
| Secondary Outcomes | Use of continuous lorazepam infusion4% vs. 31%Difference 27% [95% CI 14–41%]Total lorazepam required26 vs. 49 mg Difference 23 mg [95% CI 7–40] There were no differences in telemetry admission or floor ward admissionTrend toward lower median ICU or total hospitalHospital LOS: 76 hr (54–114) vs 118 hr (47–190) ICU LOS: 34 hr (30-276) vs 94 hr (43–134) |
| Adverse Effects | No differences in incidence of intubation, seizure, mechanical restraints, and bedside sitter.There were no falls or mortality reported in either group. |
Discussion
Strengths
- Randomized
- Prospective
- Clinical relevant study outcomes
Limitations
- Formal sample size analysis was not done
- Small sample
- Single Center
- Delirium, respiratory depression, EtOH level, and hypotension were missing from analysis
Takeaways
- Phenobarbital is an option as adjunct to benzodiazepine for AAWS
- 10 mg/kg did not lead to significant increase in adverse effects compared to standard of care
Alcohol withdrawal syndrome in critically ill patients: Protocolized versus Nonprotocolized management.
Duby JJ et al. J Trauma Acute Care Surg. 2014 Dec;77(6):938-43
| Objective | to compare patient outcomes in critically ill patients with AWS, regardless of their admission ICU diagnosis, that were treated with this protocolized approach versus a non-protocolized approach. |
| Design | Retrospective pre-post study. |
| Population | 135 patients with suspected acute alcohol withdrawal syndrome admitted to the ICU |
Design
| Inclusion/Exclusion | I: > 18 year old with suspected acute alcohol withdrawal syndrome admitted to ICU E: Patients with severe brain injury—defined as persistent Glasgow Coma Score < 8 |
| Intervention | Pre-Protocol:Typically received continuous infusions or scheduled doses of BZDs per physician preference Post-ProtocolEscalating doses of diazepam and phenobarbital according to an AWS protocol |
| Outcomes | Primary: ICU length of stay Secondary: Mean and median BZD use, mean and median phenobarbital use, duration of sedation, requirement for mechanical ventilation (MV), ventilator-free days, and requirement for MV due to AWS |
Baseline Characteristics
| Pre (n = 60) | Post (n = 75) | P Value | |
| Age | 55.7 ± 8.7 | 50.7 ± 13.8 | 0.03 |
| Male | 81.6% | 81.3% | 1.0 |
| History of Alcohol Withdrawal | 40% | 30.6% | 0.28 |
| History of Psychosis | 10% | 12% | 0.78 |
| History of Delirium Tremens | 10% | 4% | 0.19 |
| History of Seizure | 18.3% | 21% | 0.83 |
| Mean SOFA score on admit | 6.1 ± 3.7 | 3.9 ± 2.9 | 0.0004 |
| Mean blood alcohol level on admit (mg/dL) | 135 ± 156 | 134 ± 140 | 0.56 |
Results
| Primary Outcome | ICU LOS: Pre vs Post Protocol9.6 ± 10.5 vs 5.2 ± 6.4 (P-value 0.0004) |
| Secondary Outcomes | Time on Ventilator (days)5.6 ± 13.9 vs 1.31 ± 5.6 (P-value < 0.0001) Ventilator-free days21.3 ± 9.5 vs 26.3 ± 5.6 (P-value 0.0004) Intubation due to AWS 13 (22%) vs 4 (5%) (P-value < 0.001) Need for continuous sedation33 (55%) vs 18 (24%) (P-value < 0.001) Duration of sedation (days)10.8 ± 8.9 vs 3.5 ± 3.5 (P-value < 0.001) |
| Adverse Effects | Death7 (12%) vs 2 (3%) (P-value 0.07) |
Discussion
Strengths
- Clinical relevant study outcomes
- Provided protocol
Limitations
- Retrospective
- Small sample
- Single Center
- Delirium, respiratory depression, and hypotension were missing from analysis
Takeaways
- Phenobarbital is an option as adjunct to benzodiazepine for AAWS
- Protocol utilizing adjunct phenobarbital may reduce ICU LOS
Tidwell WP, Thomas TL, Pouliot JD, Canonico AE, Webber AJ. Treatment of Alcohol Withdrawal Syndrome: Phenobarbital vs CIWA-Ar Protocol. Am J Crit Care. 2018;27(6):454-460.
- Objective
- To compare a symptom-triggered benzodiazepine protocol to a phenobarbital protocol for the treatment of alcohol withdrawal
- Design
- Retrospective cohort study of a 42-bed medical ICU in a private teaching hospital in Nashville Tennessee
- Inclusion: Medical ICU patients admitted from January 1, 2016 through June 30, 2017 and treated for the onset or prevention of AWS
- Exclusion: Received CIWA-Ar-based treatment for >24 hours before starting phenobarbital protocol; received no doses of either protocol; pregnant; had phenobarbital as a documented outpatient maintenance medication
- Intervention and Comparator
- Phenobarbital taper protocol including lorazepam prn for agitation – initial phenobarbital dose 64.8 mg PO TID-260 mg IV based on history of delirium tremens, n=60
- CIWA-Ar-based lorazepam protocol, n=60
- Outcomes (Phenobarbital protocol vs CIWA-Ar protocol)
- Use of phenobarbital protocol was associated with a statistically significant ↓ in ICU LOS (2.5 vs 4.4, p <0.001)
- Use of phenobarbital protocol was associated with a significantly ↓ total hospital LOS (4.3 vs 6.9, p=0.004)
- Use of phenobarbital significantly ↓ the total lorazepam equivalents (11.3 mg vs 35.2 mg, p <0.001)
- Use of adjunctive medications, specifically dexmedetomidine, was much lower in the phenobarbital group (7% vs 17%, p=0.002)
- Limitations
- Small sample size, single-center, retrospective design
- High incidence of comorbid conditions may have affected need for mechanical ventilation
- Conclusions
- Phenobarbital-based treatment of AWS seems to provide comparable benefit to traditional benzodiazepine-based treatment
Overview of Evidence
Studies evaluating the use of Phenobarbital in Benzodiazepine in alcohol withdrawal
| Author and Year | Research Design and Sample size | Intervention | Results | |
| Nisavic, 2019 | Retrospective observational/ n=562 | Benzodiazepine only fixed dosingPhenobarbital- Based Protocol (IM load + PO taper) | No difference in AWS-related seizures, ICU admission, over-sedation, LOS, and hallucinations ↑ Delirium in BZD group In BZD→PB crossover pts, PB led to rapid improvement of BZD resistant AWS symptoms | |
| Sullivan, 2018 | Retrospective observational/ n=209 | Benzodiazepine only CIWA- Protocol PB + Benzodiazepine CIWA Protocol | No difference in ICU admission, intubation, hypotension, ED LOS, CIWA score at ED discharge PB group had ↓ hospital LOS and Max CIWA score at 24 hrs | |
| Rosenson, 2013 | Retrospective cohort analysisn=102 | PB 10 mg/kg IV x1 + PRN benzodiazepines Placebo + PRN benzodiazepines | PB had ↓ ICU admission PB had ↓continuous infusion lorazepam PB had ↓ total lorazepam requirements No difference in ICU or hospital LOS | |
| Duby et al (2014) | Retrospective, pre-post studyn = 135 | Nonprotocolized BZD versus symptom- triggered, protocolized dose escalation of diazepam and phenobarbital | ICU LOS was significantly lower with protocolized delivery (5.2 ± 6.4 days vs 9.6 ± 10.5 days, P = 0.0004); significantly fewer intubations for AWS with protocolized delivery (5% vs 22%, P < 0.001). Protocolized delivery resulted in significantly less time on the ventilator, more ventilator free days, less need for continuous sedation, and shorter duration of sedation | |
| Ibarra, 2019 | Retrospective observational/ n=78 | Lorazepam protocol only (LZP) PB x 1 + LZP protocol (PB+LZP) | No difference in daily lorazepam requirements or hospital LOS PB+LZP group had ↑ pts d/c within 72 hrs No patient in PB group experienced intubation or hypotension | |
| Nelson, 2019 | Pre-post observational/ n=300 | IV diazepam alone (DZP) IV LZP + IV PB (LZP + PB) IV PB alone (PB) | No difference in ICU admission, ICU LOS, and need for intubation. PB associated with ↑ ED LOS but ↓ BZD requirements | |
| Sullivan, 2018 | Retrospective observational/ n=209 | BZD only CIWA- Protocol PB + BZD CIWA Protocol | No difference in ICU admission, intubation, hypotension, ED LOS, CIWA score at ED discharge PB group had ↓ hospital LOS and Max CIWA score at 24 hrs | |
| Hendey, 2011 | Prospective, randomized, double-blind trial n=44 | PB 260 mg IV ×1, 130 mg IV PRN Lorazepam 2 mg IV PRN + PRN chlordiazepoxide | PB and LZ both reduced the average CIWA-Ar score from baseline to discharge No difference in ED LOS and hospital LOS | |
| Young, 1987 | Prospective, uncontrolled trial n=62 | 260 mg IV ×1 then 130 mg IV until clinical end point of light sedation | Safe discharge from ED was achieved in 92% of patients Average ED LOS was 3 h, 47 min No discharged patients returned to ED during the following week Adverse effect in 6% of patients (none were admitted to hospital): 1 hypotension, 1 ataxia, 2 lethargy after final bolus doses |
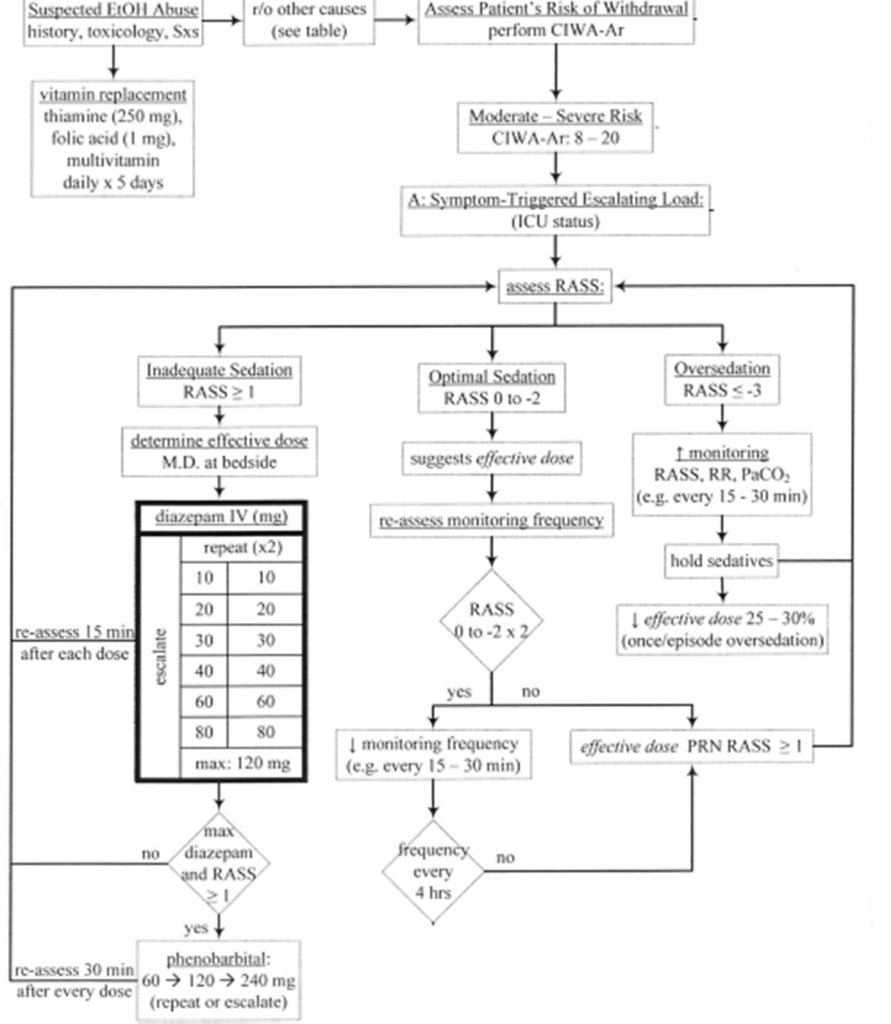
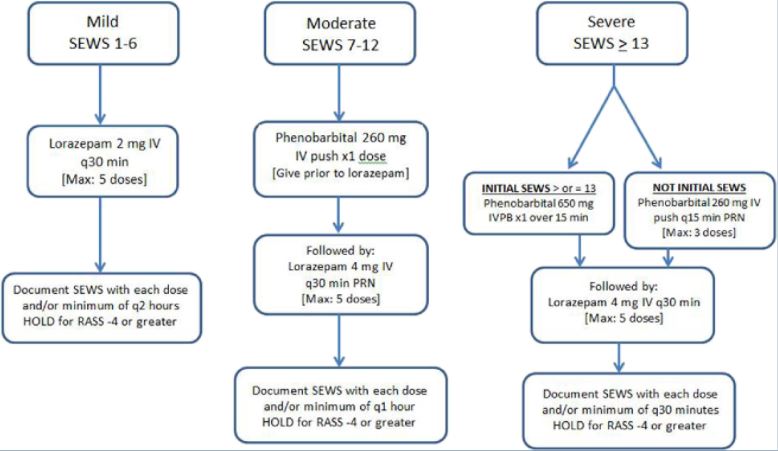
Phenobarbital Protocols