Heparin
- Mechanism of Action:
- Binds to antithrombin III, converts to an active form, and inhibits thrombin and factor Xa
- Dosing & Administration:
- IV 50- to 70-U/kg then infusion based on reperfusion plan
- Adverse Effects:
- Bleeding & thrombocytopenia
- Contraindications:
- Severe thrombocytopenia & active bleeding
- Clinical Pearls & Practical Considerations:
- Heparin has a short half-life and its effect can be rapidly reversed by protamine sulfate.
- Heparin can also cause heparin-induced thrombocytopenia (HIT), an immune-mediated reaction that can lead to thrombosis. HIT can occur 5 to 10 days after initiation of heparin therapy.
- Monitoring: aPTT, INR, CBC, platelet count
LMWH
Bivalirudin
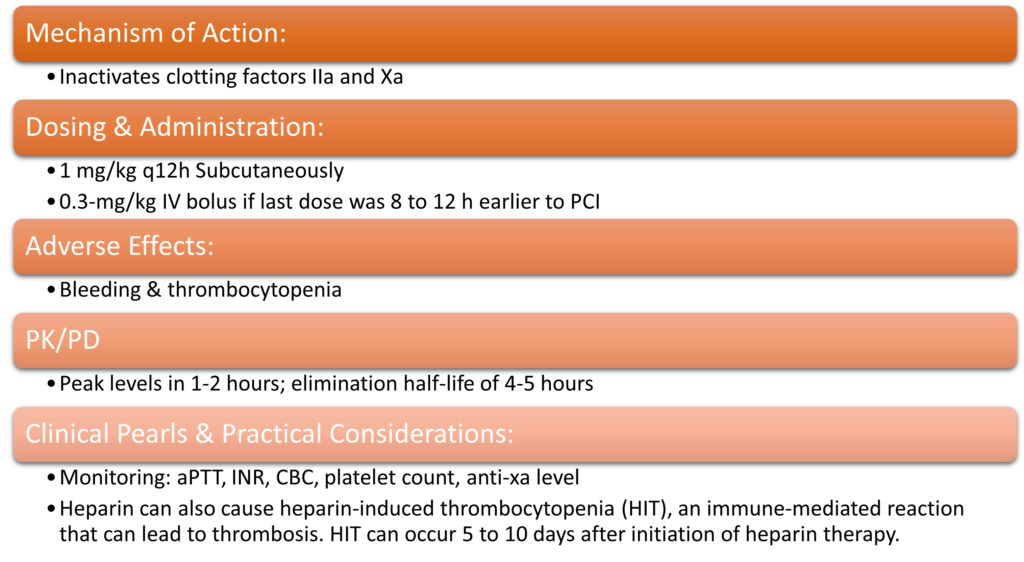
- Mechanism of Action:
- Binds to thrombin, preventing thrombin-mediated platelet activation and thrombus formation
- Dosing & Administration:
- 0.75 mg/kg IV bolus followed by infusion of 1.75 mg/kg/hr
- Reduce infusion to 1 mg/kg/h with estimated CrCl <30 mL/min
- Adverse Effects:
- Bleeding, Hypotension, Nausea, & Anaphylaxis
- PK/PD
- Plasma half-life of 30 minutes; metabolized by liver
- Clinical Pearls & Practical Considerations:
- It is often used as an alternative to heparin plus GPIIb/IIIa inhibitors in patients with high bleeding risk.
- May be preferred in those with high risk of bleeding
- There is no specific reversal agent for bivalirudin
Anticoagulant Drug Chart
| Drug | Mechanism of Action | Dosage and Route of Administration | Onset of Action | Elimination Half-life | Adverse Effects |
| Heparin | Indirect thrombin | 50-70 unit IV bolus, followed by continuous infusion | Immediate | 1-2 hours | Bleeding, HIT, thrombocytopenia, hypersensitivity, hyperkalemia, osteoporosis |
| Enoxaparin | Indirect Factor Xa | 1 mg/kg Subcutaneous | 3-5 hours | 4-7 hours | Bleeding, thrombocytopenia, HIT, hyperkalemia, hypersensitivity, osteoporosis |
| Bivalirudin | Direct thrombin | 0.75 mg/kg IV bolus followed by infusion of 1.75 mg/kg/hr | Immediate | 25 minutes | Bleeding, anemia, hypotension, thrombocytopenia, angina, dyspnea, allergic reaction |
| Fondaparinux | Indirect Factor Xa | Subcutaneous | 3 hours | 17-21 hours | Bleeding, thrombocytopenia, anemia, injection site reaction, hypersensitivity, caution in renal impairment |
AHA STEMI Guidelines- Anticoagulants Prior to PCI
| Drug | Recommendation | LOE | COE |
| UFH | •With GP IIb/IIIa receptor antagonist planned: 50- to 70-U/kg IV bolus to achieve therapeutic ACT of 200 to 250 s. •GP IIb/IIIa receptor antagonist planned: 70- to 100-U/kg bolus to achieve therapeutic ACT is 250 to 300 s (HemoTec device) or 300 to 350 s (Hemochron device). | I | C |
| Bivalirudin | 0.75-mg/kg IV bolus, then 1.75-mg/kg/h infusion with or without prior treatment with UFH. •An additional bolus of 0.3 mg/kg can be given if needed. •Reduce infusion to 1 mg/kg/h with estimated CrCl <30 mL/min | IIa | B |
| Preferred over UFH with GP IIb/IIIa receptor antagonist in patients at high risk of bleeding | IIa | B | |
| Fondaparinux | Not recommended as sole anticoagulant for primary PCI | III | Harm B |
